Posted byarmsem01Leave a commenton Chemistry is Like Cooking…Just Don’t Lick the Spoon!EditChemistry is Like Cooking…Just Don’t Lick the Spoon!
Hello and welcome to the Jameson group blogpost for Summer ’21! This summer Erin McGrath (’23) has joined veteran Emma Armstrong (’21) in our mission to prepare and study new complexes of 1,2-dioxime ligands.

These ligands are well known as building blocks of a class of coordination complexes known as cobaloximes. These complexes, very well studies as model complexes of the co-enzyme vitamin B12 , have been recently rediscovered by chemists as components in catalysts that use sunlight to convert water to hydrogen and catalysts that make carbon-carbons bonds.
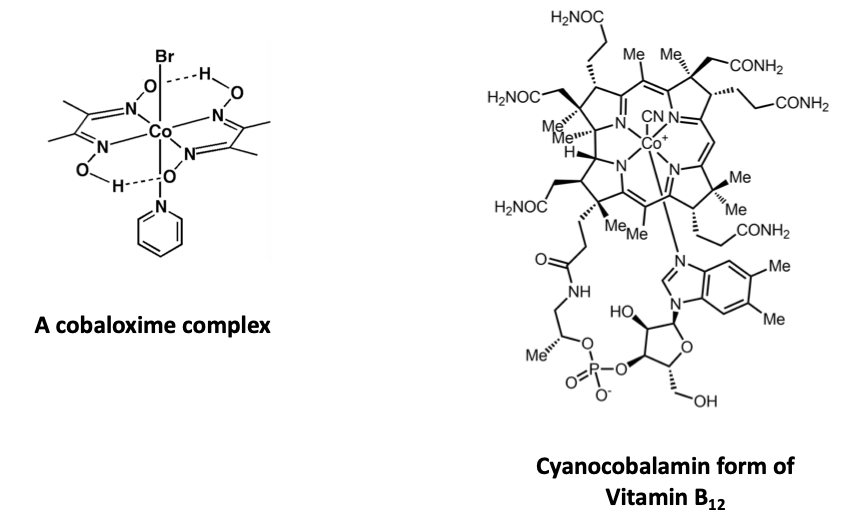
Note that, despite the complex overall structure of vitamin B12, the structure around the cobalt atom in both molecules is very similar.
Everyone will remember the oxime functional group from the second semester of organic chemistry ;). If you put two oximes next to each other on a carbon chain (1,2), they can act as a “bidentate” ligand – in other words they “bite” onto a metal two times. 1,2-dioximes are very old molecules (they were discovered in the 1880s) and they have been used to bond to metal ions since 1905.
The C=N double bond is incapable of free rotation (as you’ll remember from both CH107 and CH203) and this gives rise to the possibility of isomers. With a symmetric dioxime (by far the most common type), there are three isomer possibilities. With an unsymmetric dioxime, a fourth isomer is possible. By far the most common isomer found bonded to metals is the a-isomer, which bonds to metal ions through both nitrogens, to form a five-membered chelate ring. The g-isomer is capable of forming six-membered chelate rings by bonding through one N and one O. This type of coordination complex is exceedingly rare.

In fact, you might expect that when preparing 1,2-dioximes from 1,2-diketones (by reacting with hydroxylamine) a statistical mixture (a:g:b = 1:2:1) would be obtained. In fact, the a isomer is often obtained in 70-95% yield (as opposed to the expected 25% maximum yield). This may be why so many so isomers of the g-isomer have been prepared. The synthesis of 1,2-dioximes typically takes place in mild acid. Raising the pH of the reaction can lead to different ratios of isomers.
Despite the thoroughly mined vein of 1,2-dioxime coordination chemistry (there are almost 5500 references to metal complexes of the a-isomer of 1,2-dioximes), we feel there a few nuggets left to uncover. Our hope is that these we make some new complexes that might find use in the more recent catalytic applications of cobaloximes.
Cobaloxime complexes can be varied in the dioxime ligand, the neutral axial ligand or the anionic axial ligand.

Emma is working on preparing cobaloxime complexes of camphorquinone dioxime, a chiral dioxime ligand. Chiral complexes of cobaloxime have not been made before. The ligand itself is an interesting story. It exists as four C=N double bond isomers, since the ligand is unsymmetric. All four isomers were prepared, separated and the structures correctly assigned (taking into account that the regiochemistry of the Beckmann rearrangement was originally misinterpreted) by Forster just after 1900. This achievement was remarkable considering the most sophisticated instruments available at the time gave no clues about structure. We are interested in the b-isomer, which will give N,N-bonded complexes such as those found in cobaloximes. It strikes us as odd that, despite the flurry of metal complexes reported for the a-, b-, and d-isomers, no cobaloxime complexes of the b-isomer were ever reported.

Cobaloxime complexes of b-camphorquinone dioxime provide their own challenges of isomers. The ligand can orient itself in the complex is three different ways: two different ways with the dimethylcarbon bridge (circled) syn (in the same direction) and one with the dimethylcarbon bridge anti (in the opposite direction). Luckily, these three isomers may be distinguished by NMR. Emma has isolated all three isomers for one such combination of L and X. This was a painstaking task requiring some very careful column chromatography.

Erin is working on cobaloximes with new variations in the anionic ligand X. While the Co-C bond of cobaloximes has been very thoroughly studied, there is only passing mention of cobaloximes of alkynes. This is despite the fact that metal-alkyne bonds are generally the most stable between metals and hydrocarbons. Erin’s initial attempts gave some clues about why cobaloxime-alkyne complexes have not been thoroughly studied. Among many failed reactions, she was able to prepare a cobaloxime-alkyne complex in about 10% yield!!! Not great, but enough to characterize and even grow some high-quality crystals (see 2019 blog post). Erin’s still got a few weeks left and a few more tricks up her sleeve. We can definitely get that yield up to 20%!!!
The pandemic forced us out of the lab for an extended period of time. What do you do if you are a synthetic chemist with a Jones for mixing and pouring and heating? For adding a little of this and a little of that and see what you get? You turn to the kitchen! Because cooking is a lot like chemistry – except you CAN lick the spoon!
A custom in the Jameson household is to watch the European tennis championships (French Open and Wimbledon) on Sundays along with a breakfast of French toast and strawberries. This can be not much fun for the cook, since French toast, for a hungry crew, needs to be cooked in several shifts. In the lab, the Jameson group is partial to simple reactions, that make surprisingly interesting molecules, at a large scale. Baked French toast is just such a “reaction”, that frees the cook up to watch tennis.
“Experimental”
The egg custard is a mixture of egg and milk in a ratio of 10 eggs/1.5 cup milk. (This is a more egg-rich custard than normally used for French toast.) The best bread is a denser and crustier, rather than soft. Dense, crusty bread will take longer to soak up the custard, so the custard-bread mix should be prepared the night before. Grease a 13 x 9 inch baking dish with butter. Slice the bread generously (~1 inch), soak each slice in the custard and “tile” the slices in the baking dish. Tiling will allow for more slices and a larger yield!! Pour the remaining custard over the bread and allow the bread to absorb the custard overnight in the fridge. The “puddles” of custard will be absorbed by the bread overnight. The next morning, sprinkle the top of the French toast with brown sugar and chopped pecans. Bake at 350 F for 1 hour to give a crisp French toast with nicely caramelized sugar (mmmmm…). Accompanying the French toast with fresh strawberries (folded with a bit of brown sugar to make a syrup) will ensure that this synthesis passes muster with the referees.

In Emma’s house (both during a pandemic and during normal times), Friday night pizza and ice cream is a staple. You won’t see any take-out pizza boxes in sight, though. Her pizza is made from scratch! During the summer of 2020, Emma’s backyard garden had an abundance of zucchini and cherry tomatoes. Using the fresh summer produce in addition to other pantry staples, Emma’s created her new favorite pizza recipe. The cooking of this pizza really highlights Maillard Reactions which result in caramelized sugars and proteins.
“Experimental”
For this recipe, Emma starts by doing all of the prep-work like cleaning, cutting, and sauteing vegetables. Zucchinis are cut into thin (less than a ¼ inch) rounds. Baby portabella mushrooms can be diced or sliced into smaller pieces. Onions are cut into ¼ inch ribbons and placed in a generously oiled sauté pan on medium low heat. The onions are stirred occasionally and left to soften and caramelize. The onion caramelization process usually takes about 20-30 minutes (try not to be tempted to crank the heat up because that will burn your onions before they fully reduce and get sweet). Generally speaking, Maillard Reactions are organic chemical reactions that result in “cooked” food, or food that has be caramelized, reduced, or browned. The resulting products of Maillard Reactions are chemicals that change of enhance the flavor of the food. Cherry tomatoes, as many as you would like, are cut in halves. Parmesan cheese can be finely grated, sharp provolone cheese can be thinly sliced, and mozzarella can be coarsely shredded. Lastly, preheat the oven to 450°F. Prep work is complete! This is a lot like measuring reagents used in lab for a chemical synthesis. Cooking and chemistry both go more smoothly when you have everything you need ready for use.
On a lightly greased pizza stone or baking pan, press out a hefty ball of pizza dough (Emma loves buying Aldi’s pizza dough from the refrigerated section near the cheeses) gently with your fingertips to your desired size and shape. Once the dough is spread evenly, the sauteed onion mixture can be applied. Next, generously sprinkle dried oregano, parsley, and red chili flake to your taste. The thin zucchini rounds go over the onions in an even layer followed by pieces of provolone cheese and tomatoes. To top your pizza off, mozzarella and parmesan cheese can be applied as minimally or as liberally as preferred. The pizza is cooked for 20-25 minutes, or until the crust is golden brown and the cheese is bubbling.

On some of the mental health days or snowy mornings some of the best things to make was a warm breakfast. After finding a heart shaped waffle maker before moving into my apartment I knew that there would be many mornings of apartment brunches. I began bribing my roommates with Nespresso drinks and chocolate chips to try the gluten free waffles that I whipped up. The first time was impulsive and called for improvising for half of the ingredients but by the end of the semester there was no need for any more bribing.
“Experimental”
Before getting out any of the ingredients it’s best to start preheating your waffle iron if you are as impatient as I am. Start by adding 2 cups of any all-purpose Gluten-free flour, I usually use Krusteeze in one large bowl along with about one tablespoon of granulated sugar and one teaspoon of baking powder. In a separate bowl add 2 large eggs, about 1 teaspoon of vanilla, 1 and ¾ cup of milk or almond milk, and half a cup of vegetable oil. Mix the bowl of wets together then add that to the dry ingredients. Chocolate chips are always a great idea to add to the batter or mini m&ms for a more colorful look. Then you can spray your waffle iron with non-stick spray and then add your waffle mix to the iron. It should be about 5-10 minutes before they can be flipped and then taken off the waffle iron. minutes to be rWhen they finish they may be a little flatter than non-gluten free ones but don’t worry they will taste just as good if not better. They are best paired with clementine quarters or home fries and coffee.






